Insufficient Research On Cervical Cancer Vaccine Raises Concern
August 12, 2015 06:42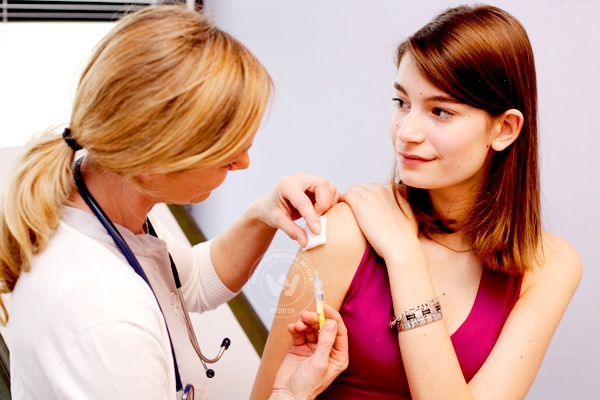
(Image source from: })
Recently, Indian Government has planned to introduce cervical cancer vaccine in the universal immunization program. But several civil society organizations and public health groups raised concerns over the hurried license of cervical cancer vaccine in India, on the basis of grossly insufficient research.
They urged Health Minister JP Nadda through a communication that the plan to include human papilloma virus (HPV) vaccine against cervical cancer should be dropped from the universal immunization program. They highlighted insufficient clinical trials of vaccine on Indian population and raised concerns on its safety and efficacy.
Nearly 70 representatives belonging to leading public health groups and women's groups, health and women's rights activists and health researchers have signed the memorandum sent to Nadda.
The memorandum says, "We are extremely concerned about the long-term safety and efficacy of the HPV vaccines - Gardasil and Cervarix - and strongly feel that it would lead to serious adverse effects for its recipients. The Supreme Court is hearing the writ petitions that have raised important questions regarding the vaccine's safety and efficacy as well as its relevance and priority as a public health measure in India.”
Lack of research on cervical cancer vaccine
The emphasis is on the lack of sufficient evidence of the safety and efficacy of HPV vaccine in the Indian population. More research is needed to know more about the vaccine. Details like if a repeat dose is needed, time span for the protection from HPV infection is yet to be studied.
The memorandum said, "There is no conclusive evidence which suggests that the vaccine will protect girls from acquiring HPV and developing cervical cancer later in their lives. These vaccines have not been in use for long enough to know the level of protection they will offer to young women when they are actually exposed to the risk of HPV infection.” The letter also added that many girls suffered side effects from the cervical cancer vaccine, while nearly 7 died after HPV vaccination.
The allegation by Public health organizations like Jan Swasthya Abhiyan and Sama is that cervical cancer vaccines were hurriedly licensed in India, taking into account the result from insufficient research.
-Sumana